Radioembolization
(2) Feasibility of CT Based Tumor Dosimetry Using Multimodal Imageable Glass Eye90 microspheres®
Saturday, September 23, 2023
6:00 PM - 7:30 PM East Coast USA Time
Amit Verma, DrPh, MPH – Clinical Program Director, ABK Biomedical Incorporated; David Dobrowski, N/A – VP, Clinical Development & Regulatory Affairs, ABK Biomedical Incorporated; Adam Kulp, PhD – Clinical Engineer, MIM Software Inc.; Andrew Holden, ONZM, MBChB, FRANZCR, EBIR – Associate Professor, Radiology, Auckland City Hospital; Eric Henry, PhD – Postdoctoral Fellow, Imaging Physics, MD Anderson Cancer Centre; S Kappadath, PhD – Professor, Imaging Physics, The University of Texas MD Anderson Cancer Center; Alasdair Syme, PhD – Associate Professor, Imaging Physics, Dalhousie University/NS Health Authority; Aravind Arepally, MD FSIR – Chief Medical Officer, ABK Biomedical Incorporated
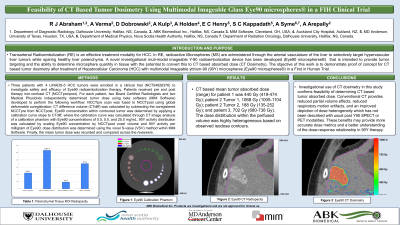
Purpose: To demonstrate proof of concept for CT based tumor dosimetry after treatment of Hepatocellular Carcinoma (HCC) with multimodal imageable yttrium-90 (90Y) microspheres (Eye90 microspheres®) in a First in Human Trial.
Material and Methods: Three patients with 4 LIRADS-5 HCC tumors were enrolled in a clinical trial (NCT04926376) to investigate safety and efficacy of Eye90 radioembolization therapy. Patients received pre and post therapy non-contrast CT (NCCTpre/post). For each patient, two Board Certified Radiologists and two Medical Physicists independently determined tumor dose using beta software (MIM Software) developed to perform the following workflow: NCCTpre scan was fused to NCCTpost using global deformable coregistration; CT difference volume (CTdiff) was calculated by subtracting the coregistered NCCTpre from NCCTpost; Eye90 concentration within contoured tumor was determined by applying a calibration curve slope to CTdiff, where the calibration curve was calculated through CT image analysis of a calibration phantom with Eye90 concentrations of 0.5, 5.0, and 25.0 mg/mL; 90Y activity distribution was calculated by scaling Eye90 concentration by NCCTpost voxel volume and 90Y activity per milligram of Eye90; dose distribution was determined using the voxel S-value (VSV) method within MIM Software. Finally, the mean tumor dose was recorded and compared across the reviewers.
Results: CT based mean tumor absorbed dose (range) for patient 1 was 440 Gy (419-474 Gy); patient 2 Tumor 1, 1068 Gy (1005-1104 Gy); patient 2 Tumor 2, 188 Gy (135-252 Gy); and patient 3, 702 Gy (680-738 Gy). The dose distribution within the tumor was highly heterogeneous based on observed isodose contours.
Conclusions: Investigational use of CT dosimetry in this study confirms feasibility of determining CT based tumor absorbed dose. Conventional CT provides reduced partial volume effects, reduced respiratory motion artifacts, and an improved depiction of dose heterogeneity which has not been described with usual post Y90 SPECT or PET modalities. These benefits may provide more accurate dose metrics and a better understanding of the dose-response relationship in 90Y therapy.
Material and Methods: Three patients with 4 LIRADS-5 HCC tumors were enrolled in a clinical trial (NCT04926376) to investigate safety and efficacy of Eye90 radioembolization therapy. Patients received pre and post therapy non-contrast CT (NCCTpre/post). For each patient, two Board Certified Radiologists and two Medical Physicists independently determined tumor dose using beta software (MIM Software) developed to perform the following workflow: NCCTpre scan was fused to NCCTpost using global deformable coregistration; CT difference volume (CTdiff) was calculated by subtracting the coregistered NCCTpre from NCCTpost; Eye90 concentration within contoured tumor was determined by applying a calibration curve slope to CTdiff, where the calibration curve was calculated through CT image analysis of a calibration phantom with Eye90 concentrations of 0.5, 5.0, and 25.0 mg/mL; 90Y activity distribution was calculated by scaling Eye90 concentration by NCCTpost voxel volume and 90Y activity per milligram of Eye90; dose distribution was determined using the voxel S-value (VSV) method within MIM Software. Finally, the mean tumor dose was recorded and compared across the reviewers.
Results: CT based mean tumor absorbed dose (range) for patient 1 was 440 Gy (419-474 Gy); patient 2 Tumor 1, 1068 Gy (1005-1104 Gy); patient 2 Tumor 2, 188 Gy (135-252 Gy); and patient 3, 702 Gy (680-738 Gy). The dose distribution within the tumor was highly heterogeneous based on observed isodose contours.
Conclusions: Investigational use of CT dosimetry in this study confirms feasibility of determining CT based tumor absorbed dose. Conventional CT provides reduced partial volume effects, reduced respiratory motion artifacts, and an improved depiction of dose heterogeneity which has not been described with usual post Y90 SPECT or PET modalities. These benefits may provide more accurate dose metrics and a better understanding of the dose-response relationship in 90Y therapy.
